Expert Labeling: Private Label Vitamins and Supplements, Pets & Skincare Products
Superior Quality Label Creation Service Options:
- Full Label Creation One-time Fee: $350.00-$150.00 per Product Template. Your Sales Manager or Private Label Supplement Creative Department will advise you on specifics for each new Full Label Creation if varied from Product Template per valued Client. Client is responsible for their label copy per FDA Labeling Guidelines. This includes all logos, center panel images, emblems, GS1 UPC's, FNSKU's as requested on the Label Project Brief or Client will be subject to an additional hourly fee of $350.00 for the creation of art per labeling guidelines and purchase of art.
- Private Label Supplement Template One-time Fee (see template options below): $225.00-$175.00 per Product Template. Your Sales Manager or Private Label Supplement Creative Department will advise you on specifics for each additional Product Template if varied from Product Template per valued Client.
- Client Supplied Center Panel Artwork One-time Fee: $200.00-$100.00 per Product Template. Your Sales Manager or Private Label Supplement Creative Department will advise you on specifics for each additional Client Supplied Center Panel Artwork if varied from Product Template per valued Client.
- Branded Company Logo with Slogan creation is available through our Marketing Solutions division for $350.00. The Client holds an option to provide logo artwork in a Vector .eps or Adobe Illustrator .ai file.
- Client Provides Label Artwork; design fees do not apply. Private Label Supplement must review label, and ultimately, Client is responsible for their label copy per FDA Labeling Guidelines.
- Once labels are on file with Private Label Supplement, label creation fees are not recurring.
- If Client elects to create their label and should Private Label Supplement provide advice on more than two rounds of label changes per FDA Labeling Guidelines www.fda.gov, then Client will be subject to an hourly Consulting Fee of $350.00. Please refer to our Terms & Conditions about label content responsibility. Private Label Supplement is here to serve our Client in a far superior manner than you have ever expected or experienced.
Full Label Creation Services:
- Private Label Supplement will send an email with a Label Project Brief confirming and suggesting text information to be included on the label once all elements are in place with your order, as discussed with your Sales Manager.
- Our Label Project Brief is meant to capture your vision of your completed label. Providing accurate and thorough information will help facilitate the process and, ultimately, the concept you are envisioning.
- Client-supplied GS1 UPC's or FNSKUs can be included on the label. All codes need to be provided at the same time as the logo and attached in the proper format when submitting the Label Project Brief (please refer to Label Creation Options #4).
- Once information is received, Private Label Supplement will present the label concept to Client within 48-72 hours (once complete information has been collected).
- One round of minimal changes from original proof submitted to Client is standard. If changes are not considered insignificant, you will be notified in advance if additional creative fees are required.
- If Client would like further revisions, additional fees will apply. Costs will vary based on modifications, and be communicated on a case by case basis.
- A final version of the label will be sent to Client, and upon written approval, Private Label Supplement will print the label.
- Clients can review print-ready proof upon request.
- Delayed responses to label proofs can cause deferment in the final production of your product.
Private Label Supplement Template:
- Client may choose one of Private Label Supplement’s label templates below.
- Client has the option of mixing and matching colors. Altering label design will fall into Full Label Creation Services option.
- Private Label Supplement will email a Label Project Brief confirming and suggesting text information be included on the label. Client will provide feedback and approval.
- Client-supplied GS1 UPC codes and FNSKU’s can be included on label. All codes need to be supplied at the same time as the logo when submitting the Label Project Brief (please refer to Label Creation Options #4).
- Once information is received, Private Label Supplement will present the label concept to Client within 48-72 hours (unless other arrangements have been made).
- One round of minimal changes from original proof submitted to Client is standard. If changes are not considered minimal, you will be notified in advance if additional creative fees are required.
- If Client would like further revisions, additional fees will apply. Fees will vary based on modifications, and be communicated on a case by case basis.
- The final version of the label will be sent to Client, and upon written approval, Private Label Supplement will print the label.
- Clients can review print-ready proof upon request.
- Delayed responses to label proofs can cause deferment in the final production of your product.
Please Choose From One of the Following (click image for larger view):
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Client Supplied Center Panel Artwork:
- Private Label Supplement will email a Label Project Brief confirming and suggesting text information be included on the label. Client will provide feedback and approval.
- Clients providing center artwork, including logo (please refer to Label Creation Options #4), must follow the "File Format" section below to ensure timely Label Creation.
- Client-supplied GS1 UPC codes and FNSKU’s can be included on the label. All codes need to be supplied at the same time as artwork when submitting the Label Project Brief (please refer to Label Creation Options #4).
- Once information is received, Private Label Supplement will present the label concept to Client within 48-72 hours (unless other arrangements have been made).
- One round of minimal changes from original proof submitted to Client is standard. If changes are not considered minimal, you will be notified in advance if additional creative fees are required.
- If Client would like further revisions, additional fees will apply. Fees will vary based on modifications and be communicated on a case-by-case basis.
- The final version of the label will be sent to Client, and upon written approval, Private Label Supplement will print the label.
- Clients can review print-ready proof upon request.
- Delayed responses to label proofs can cause deferment in the final production of your product.
Client Creates Label:
- Private Label Supplement will email a Product Worksheet with product specifications confirming and suggesting text information be included on the label once the order has been placed and payment has cleared.
- Please create the label per "File Format" instructions below and send the completed file to Private Label Supplement within 48-72 hours (to avoid deferment in the final delivery of your product).
- Private Label Supplement will advise Client if there are corrections needed on the label. Private Label Supplement and Client can determine who will make the necessary changes. Client must adhere to all FDA Labeling Guidelines in regard to label creation and requested changes.
- Should Private Label Supplement provide advice on more than 2 rounds of label changes per FDA Labeling Guidelines www.fda.gov, then Client will be subject to an hourly Consulting Fee of $350.00.
- The Client will send the final version of the label to Private Label Supplement with print approval. Private Label Supplement will then go to print.
- Client can review print-ready proof upon request.
If a Client creates and sends printed labels to Private Label Supplement, the product specifications will be provided to Client via email after the order has been processed. Private Label Supplement must review and approve the label before printing. A retain sample of each label must be kept on file for each run of product in the Private Label Supplement corporate office. The Client is responsible for supplying these retain labels. All printed labels must be sent directly to the Private Label Supplement corporate office. Client will incur shipping fees when labels have been processed into the system and then forwarded onto the manufacturer. The Client is also responsible for their label content per the FDA Labeling Guidelines found at www.fda.gov in the appropriate area of the GMP's. "Label Roll Specifications" and "Print File Specifications" are noted in detail below and will be included on the Product Worksheet.
The Client is responsible for the regulatory compliance of all labels and labeling for their products, inclusive of holding substantiation for any claims made on the product label and labeling. Private Label Supplement will review the labels for general conformance to applicable regulations and compliance with the requirements regarding formula information and composition. Private Label Supplement does not continue to review content outside the current round of copy. As the brand owner, you are solely responsible for the content of your labels. Private Label Supplement serves as a guide in the process, however, we urge you to hire a 3rd party labeling consultant or attorney who specializes in private label packaging. Please refer to our Terms and Conditions for more information.
Note: Visual colors and resolution can vary between your monitor and the actual printed label. We recommend Clear Poly Labels for most of our Skincare products. Metallic labels are also available for supplements or skincare. To avoid the appearance of Pantone variation with metallic labels, we recommend staying with simple metallic colors like silver or gold. If color and clarity are critical, please request a digital press proof prior to approval. Additional fees will apply. Your Sales Manager or our Creative Department would be happy to discuss all options with you.
Deferment in label creation and approvals can cause a delay in the final delivery of your products.
Print File Specifications:
- Label artwork must be sent in a hi-res unlocked PDF file. CMYK @ 300dpi no larger than 2MB. Or it will be returned.
- It is essential to convert your fonts to curves prior to sending Private Label Supplement your final print-ready file. This does not apply during the editing process. Please revert to point #1.
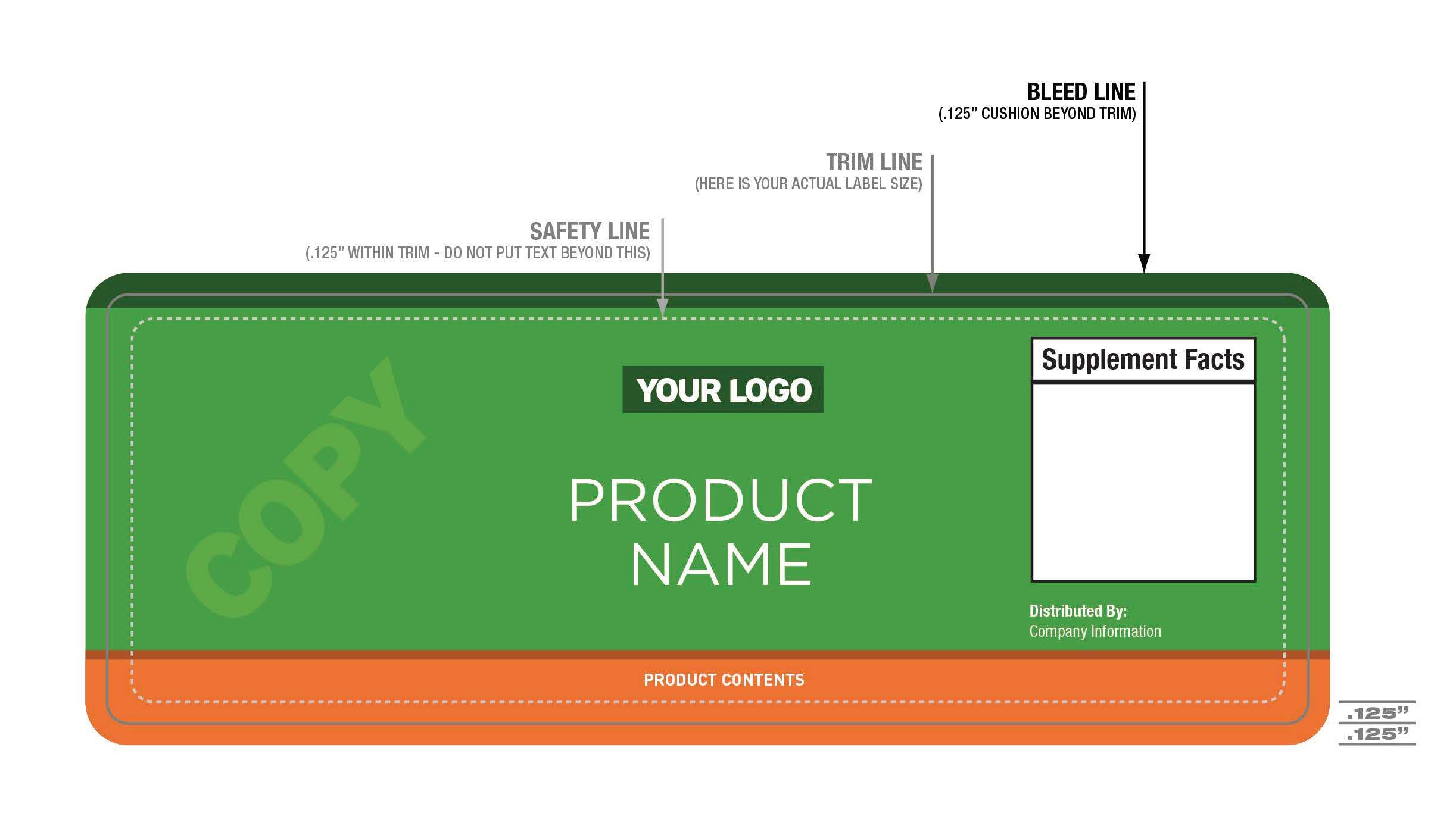
- Include a Bleed Line (.125” cushion beyond trim) beyond Trim Line (your actual label size) on all four sides of the label.
- Leave a Safety Line (.125” within trim – do not put text beyond this) on all four sides of the label.
- Please refer to the diagram below to ensure your file is set up to print correctly.
- Any variances to the "Print File Specifications" steps will be sent back to the Client for correction before proceeding.
Print Diagram Dietary Supplement (click image for larger view):
Product Categories with Corresponding Packaging Type and Label Print Size
| Dietary Supplement Bottle Size | Label Print Size |
|---|---|
| 50 cc | 1.375 x 4 |
| 75 cc | 1.625 x 5.125 |
| 100 cc | 2 x 5.5 |
| 120 cc | 2 x 5.875 |
| 150 cc, 175 cc | 2.25 x 6.25 |
| 200 cc, 225 cc | 2.5 x 7 |
| 250 cc, 275 cc, 300 cc | 3 x 7.5 |
| 400 cc, 500 cc | 3 x 8.32 |
| 650 cc | 4 x 8.75 |
| 750 cc, 950 cc, 1250 cc | 4 x 9.5 |
| 12 oz | 2.125 x 11.5 |
| 20 oz | 3 x 11 |
| 25 oz | 4 x 11.5 |
| 25 oz Canister | 4.25 x 11 |
| 1500 cc | 4.75 x 11 |
| 2500 cc | 5 x 13 |
| 2 lb, 5 lb, 1/2 Gallon | 5 x 13 |
| Pet Supplement Bottle Size | Label Print Size |
|---|---|
| 30 ml/ 1 fl oz | 1.75 x 4 |
| 60 ml/ 2 fl oz | 2 x 4.75 or 2 x 4.3 |
| 16 oz Jar (60 ct. Soft Chew) | 2.25 x 11.375 |
| 19 oz Jar (90 ct. Soft Chew) | 2.75 x 11.375 |
| 25 oz Jar (120 ct. Soft Chew) | 3.75 x 10.25 or 4.25 x 11 |
| 44 oz Jar (180 ct. Soft Chew) | 3 x 15 |
| 30 ml Clear Airless Pump | 1.875 x 4 |
| 4 Ounce Jar (60 Gram Jar) | 1.625 x 8.75 |
| 2 oz Twist Up Tube | 2.75 x 4.5 |
| 2 oz Bottle w/Black Sprayer | 2.75 x 3.875 |
| 4 oz Bottle w/Black Sprayer | 3.875 x 4.75 |
| 8 oz Bottle w/Black Sprayer | 5.125 x 5.75 |
| 12 oz Bottle w/Black Snap Cap | 5.25 x 6.75 |
| 16 oz White Bottle & Pump | 4.5 x 8.5 |
| 32 oz White Bottle & Pump | 5.625 x 10.5 |
| Skincare Bottle Size | Label Print Size |
|---|---|
| 30 ml/ 1 fl oz | 1.75 x 4 |
| 60 ml/ 2 fl oz | 2 x 4.75, 2 x 4.3 |
| 120 ml/ 4 fl oz | 2.5 x 6 |
| 2 oz Skincare Jar | 1 x 6.75 |
| 4 oz Skincare Jar | 1.5 x 8 |
| 4 oz Skincare Tottle | 3.25 x 2 |
| 6 oz Skincare Tottle | 4.75 x 1.75 |
| 15 ml Airless Skincare Pump | 1.625 x 3.625 |
| 2 oz/ 50 ml Airless Skincare Pump | 2.75 x 4.5 |
| 3 oz/ 75 ml Airless Skincare Pump | 4 x 4.5 |
| 15 ml White or Frosted Skincare Airless Pump | 1.5 x 3.25 |
| 30 ml White or Frosted Skincare Airless Pump | 1.5 x 4 |
| 50 ml White or Frosted Airless Skincare Pump | 2.75 x 4 |
| 120 ml White or Frosted Airless Skincare Pump | 4.5 x 4.875 |
| 15 ml Acrylic Skincare Jar | .5 x 7.25 |
| 60 ml Acrylic Skincare Jar | 1.25 x 7.5 |
| Airless Pump 1.7 oz/50 ml | 2 x 5.875 |
| 4 oz Spray Bottle | 2.5 x 5.75 |
Label Roll Specifications:
- 1/8" Bleed
- Rewind Position 4 (Left Side First Off)
- 3” Core
- 12” Maximum Roll
- Minimum 1/16” Gap Between Labels